Indications and Limitation of Use: ADYNOVATE® [Antihemophilic factor (Recombinant), PEGylated] is a human antihemophilic factor indicated for use in children and adults with hemophilia A for the control and prevention of bleeding episodes. ADYNOVATE is not indicated for the treatment of von Willebrand disease.1
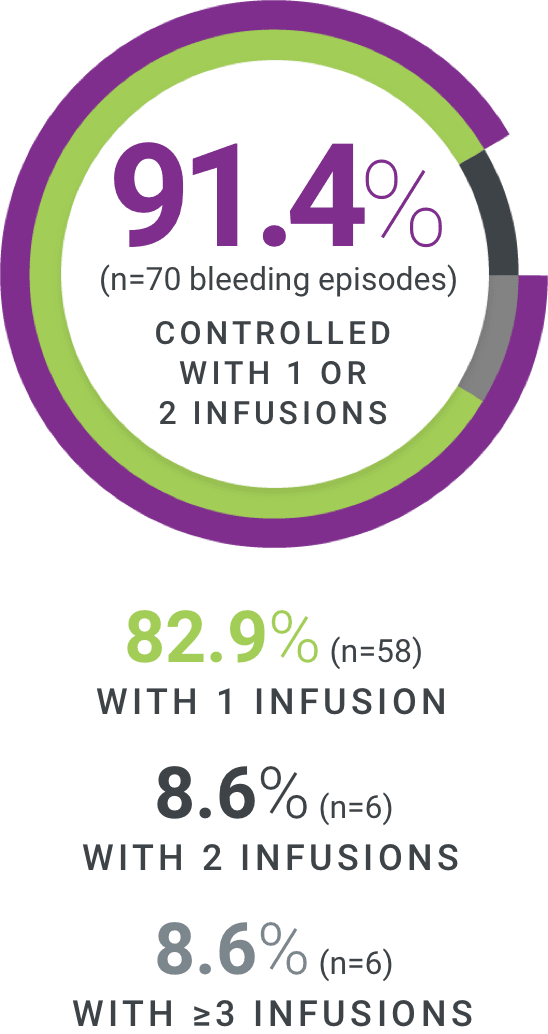
†Excellent defined as full relief of pain and objective signs of bleeding cessation; Good defined as definite pain relief and/or improvement in signs of bleeding.
CONTRAINDICATIONS: Prior anaphylactic reaction to ADYNOVATE, to the parent molecule (ADVATE [Antihemophilic Factor (Recombinant)]), mouse or hamster protein, or excipients of ADYNOVATE (e.g. Tris, mannitol, trehalose, glutathione, and/or polysorbate 80).
See Detailed Important Risk Information below.
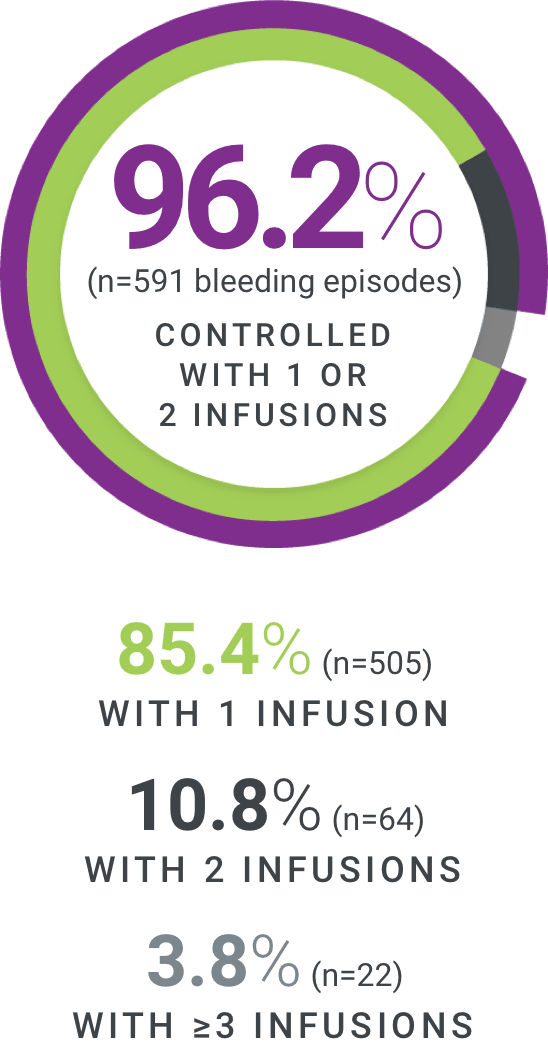
†Excellent defined as full relief of pain and objective signs of bleeding cessation; Good defined as definite pain relief and/or improvement in signs of bleeding.
Hypersensitivity Reactions: Hypersensitivity reactions, including anaphylaxis, have been reported with ADYNOVATE. Hypersensitivity reactions that can progress to anaphylaxis may include angioedema, chest tightness, dyspnea, wheezing, urticaria, pruritus, and nausea and vomiting. Immediately discontinue administration and initiate appropriate treatment if hypersensitivity reactions occur.
See Detailed Important Risk Information below.
Hemophilia A runs in Brett’s family, so it was no surprise when he tested positive for the bleeding disorder right after being born. Growing up under the watchful eye of his parents, Brett liked testing his boundaries. He confesses, “I was a kid who always wanted to do the things that I couldn’t or shouldn’t do.” At age eight, Brett set his sights on becoming a golfer. After speaking with his doctor and his parents, he got the okay to play. But there was one condition: If Brett or anyone he’s playing with thinks that he’s injured, he had to get care immediately. And it turns out, Brett became a pretty good golfer. By the age of 18, he had won 6 Junior League Golf tournaments and traveled all over the world playing.
Today, Brett is a husband and dad who strives for zero bleeds. So when his doctor told him about ADYNOVATE, Brett was very interested. His doctor prescribed infusions on Mondays and Thursdays, which works well in his busy schedule with work and family. So far, on ADYNOVATE, Brett reports he hasn't had a bleed.
![Brochure with profile of Norwood, an ADYNOVATE® [Antihemophilic Factor (Recombinant), PEGylated] hypothetical patient.](/dist/images/cta-single/cta-norwood.png)
This brochure is for healthcare providers managing patients with hemophilia A who may be reassessing their management plan.
Download brochure

The efficacy of ADYNOVATE for prophylaxis was studied in clinical trials
Get the results
ADYNOVATE has a twice-weekly dosing regimen that you can tailor to each patient
See HowYou are being directed to ADYNOVATEPro.com. The information
on this website is intended for U.S. healthcare professionals only.