ADYNOVATE has a twice-weekly prophylactic dosing schedule given on the same 2 days every week.1 Work with your patients to find a schedule that's right for them.

These are examples of what ADYNOVATE prophylaxis could look like.
![ADYNOVATE® [Antihemophilic Factor (Recombinant), PEGylated] twice weekly calendar.](/dist/images/graphs/twice-weekly.png)
†Two patients increased their dose to 60 IU/kg due to bleeding in target joints
‡Reported reasons for dose adjustment included FVIII trough levels <1%, increased risk of bleeding, and bleeding episodes
Hypersensitivity Reactions: Hypersensitivity reactions, including anaphylaxis, have been reported with ADYNOVATE. Hypersensitivity reactions that can progress to anaphylaxis may include angioedema, chest tightness, dyspnea, wheezing, urticaria, pruritus, and nausea and vomiting. Immediately discontinue administration and initiate appropriate treatment if hypersensitivity reactions occur.
See Detailed Important Risk Information below.

of patients reduced
their dosing
(69 out of 98)3
In a clinical study patients 12 years and older were able to reduce the frequency of prophylaxis dosing from their pre-study prophylaxis dosing regimen by 30% or more, which is approximately 1 fewer infusion per week.3
| children and adults (≥12 years) |
children and adults (<12 years) |
|
| DOSE | 40-50 IU/KG | 55 IU/KG with a maximum of 70 IU/kg |
| Frequency of doses |
twice weekly |
twice weekly |
Adjust the dose and dosing intervals based on the patient’s clinical response.
Because clearance (based on per kg body weight) has been demonstrated to be higher in children (<12 years), dose adjustment or more frequent dosing based on per kg body weight may be needed.1
This information is provided as a guide for dosing ADYNOVATE for the on-demand treatment and control of bleeding episodes. Maintain plasma factor VIII activity level at or above the described plasma levels (in IU per dL or % of normal) outlined in the table below.1
| Type of bleeding |
MinorEarly hemarthrosis, mild muscle bleeding, or mild oral bleeding episode |
ModerateMuscle bleeding, moderate bleeding into the oral cavity, definite hermathroses, and known trauma |
MajorSignificant gastrointestinal bleeding, intracranial, intra-abdominal or intrathoracic bleeding, central nervous system bleeding in the retropharyngeal or retrioperitoneal spaces or iliopsoas sheath, fractures, head trauma |
|---|---|---|---|
| Target Factor VIII level (IU/dL or % of normal) |
20-40 | 30-60 | 60-100 |
| Dose§(IU/kg) | 10-20 | 15-30 | 30-50 |
| Frequency of dosing(hours) |
12-24 | 12-24 | 8-24 |
| Duration of therapy |
Until the bleeding is resolved | ||
§Dose (IU/kg) = Desired factor VIII rise (IU/dL or % of normal) x 0.5 (IU/kg per IU/dL).
Please see full Prescribing Information for additional details on dosing.
This information is provided as a guide for dosing ADYNOVATE during surgery. Consideration should be given to maintain a factor VIII activity at or above the target range outlined in the table below.1
| Type of surgery |
MinorIncluding tooth extraction | MajorIntracranial, intra-abdominal, or intrathoracic surgery, joint replacement surgery |
|---|---|---|
| Factor FVIII level required (IU/dL or % of normal) |
20-40 | 60-100 |
| Dose(IU/kg) | 10-20 | 30-50 |
| Frequency of dosing(hours) |
Within one hour before surgery Repeat after 24 hours if necessary |
Within one hour before the operation to achieve 100% activity. Repeat every 8 to 24 hours (6 to 24 hours for patients <12 years of age) to maintain FVIII activity within the target range |
| Duration of therapy |
Single dose or repeat as needed until bleeding is resolved | Until adequate wound healing |
Please see full Prescribing Information for additional details on dosing.
With 7 available dosage strengths, treatment with ADYNOVATE can be personalized to meet each individual patient's needs.1
ADYNOVATE is a lyophilized powder in single-dose vials. The actual factor VIII potency is labeled on each ADYNOVATE vial to ease the identification of doses. This may be more or less than the nominal vial potency/content.1
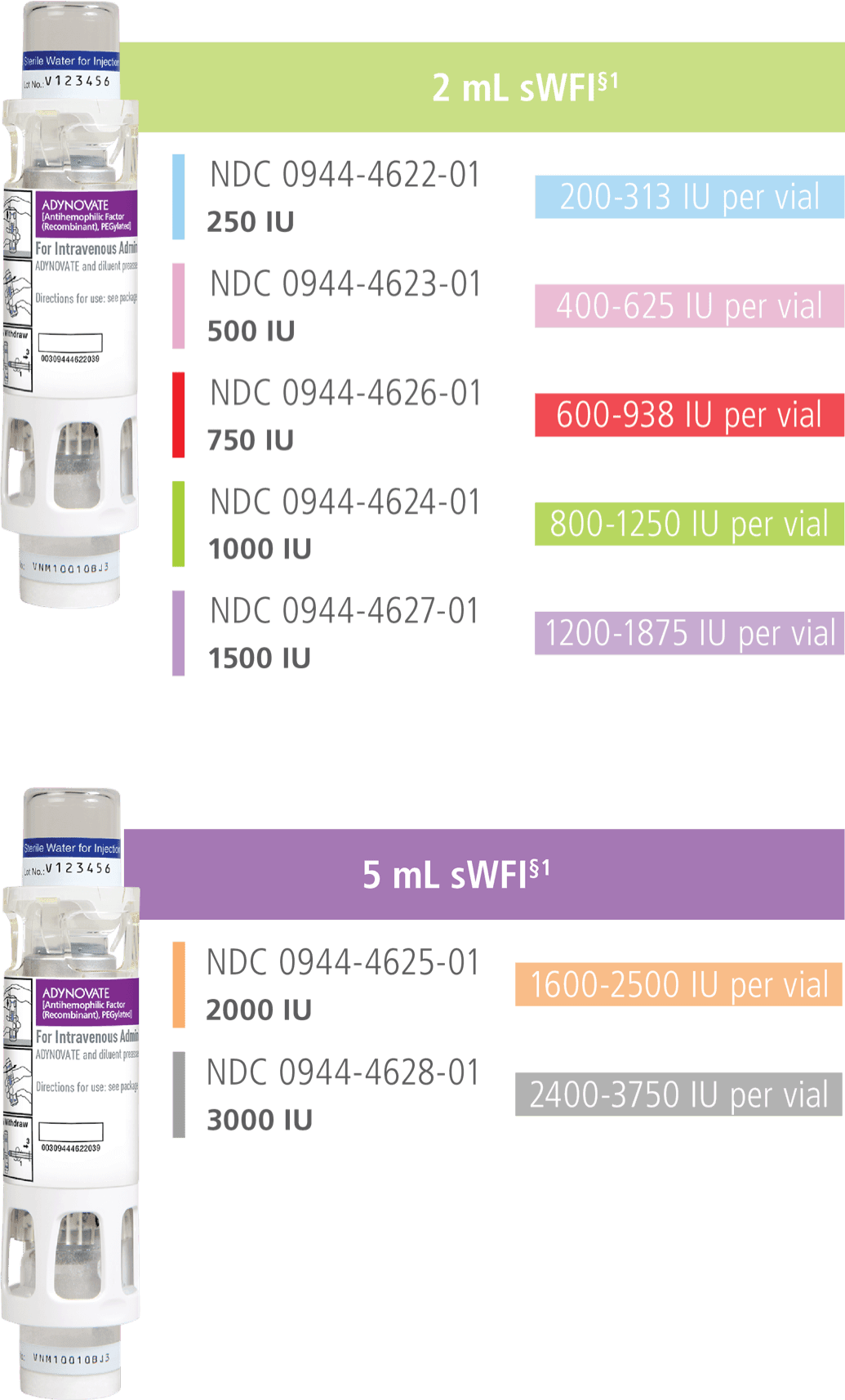
§ADYNOVATE in a BAXJECT III® system is packaged with 2 mL and 5 mL of Sterile Water for Injection (sWFI), one Terumo Microbore Infusion set (2 mL only), one full Prescribing Information insert, and one patient insert.
IU per vial/NDC code.

Easy one-step activation for reconstituting with ADYNOVATE
Watch the How-To Video
Request a local representative to answer your questions about Takeda products and programs
Request NowYou are being directed to ADYNOVATEPro.com. The information
on this website is intended for U.S. healthcare professionals only.